Poison centre notifications for hazardous mixtures: only 3 months to go before the end of the transitional period!
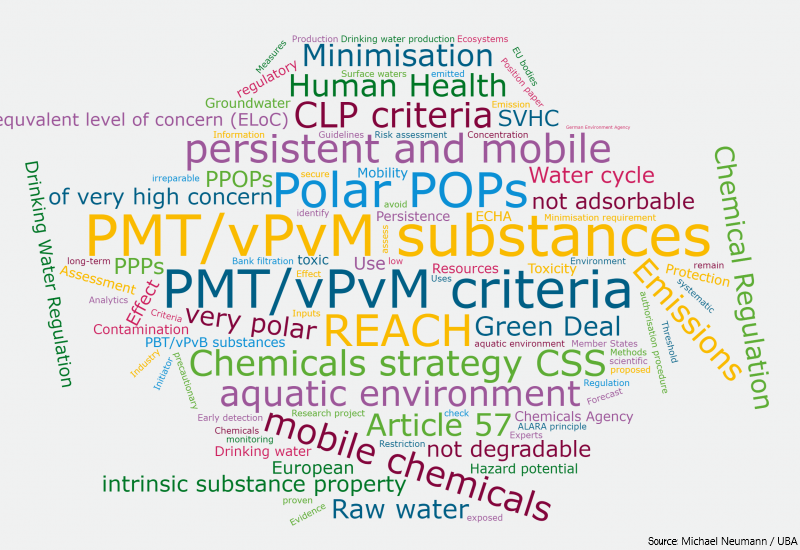
The CLP regulation (Reg. (EC) 1272/2008, article 45 and Annex VIII) requires information on mixtures classified as hazardous. This applies in particular to mixtures with physical or health hazards. Notifications must be sent to the poison centres of European Member...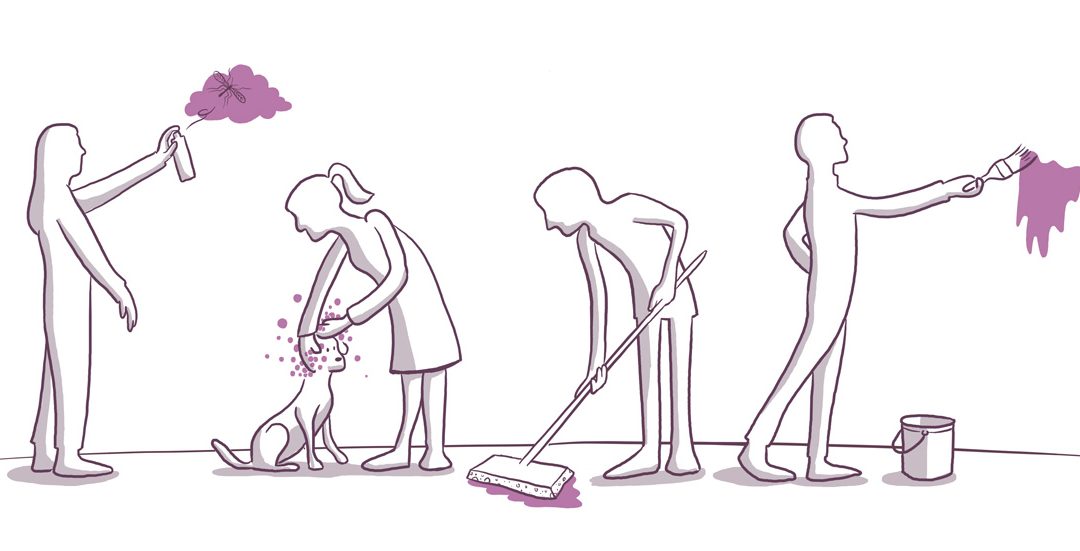
(R)EVOLUTION IN THE APPLICATION AND MANAGEMENT OF SAME BIOCIDAL PRODUCTS
The 104th meeting of the Competent Authorities (CA) took place on June 19 and 20, 2024. During this meeting, the European Commission presented its intention to review Regulation (EU) 414/2013 concerning the procedure for the authorization of SAME biocidal products. In...
Poison Centre Notification: the end of the transitional period for mixtures for industrial use!
Article 45 and Annex VIII of the Regulation (EC) 1272/2008 (CLP) require the transmission of information on mixtures classified as hazardous based on their physical or health effects to the poison control centres in the EU Member State(s) where they are placed on the...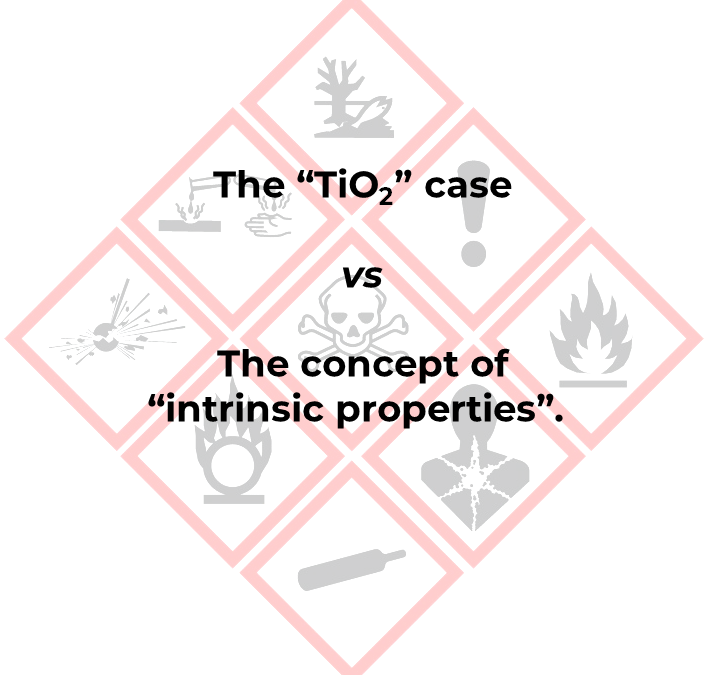
From TiO2 and beyond – Consequences of the judgment of the Court of Justice of the European Union.
On November 23, 2022, the Court of Justice of the European Union rendered a judgment annulling part of Regulation 2020/217. The cancellation concerns the harmonized classification and labeling of titanium dioxide (Carc 2, H351), including the hazard statements EUH 211...